Periodic Trends of the Elements: Tutorial
Warm-Up
The properties of an element depend on the...

Chemistry, 13.11.2020 21:20 princessss30188
Periodic Trends of the Elements: Tutorial
Warm-Up
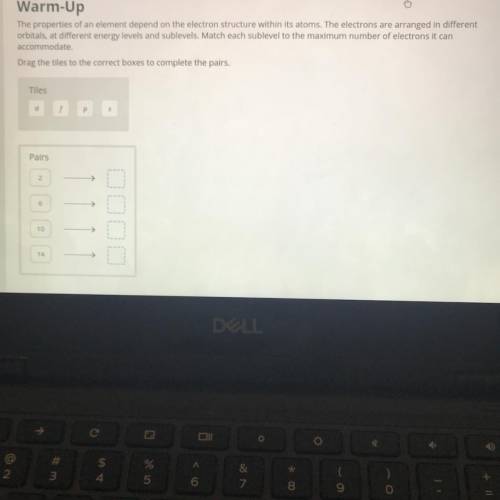
The properties of an element depend on the electron structure within its atoms. The electrons are arranged in different
orbitals, at different energy levels and sublevels. Match each sublevel to the maximum number of electrons it can
accommodate.
Drag the tiles to the correct boxes to complete the pairs.
Tiles
d
Pairs
2
6
10
14

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1


Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

You know the right answer?
Questions


Physics, 07.11.2019 19:31

Mathematics, 07.11.2019 19:31

Mathematics, 07.11.2019 19:31



World Languages, 07.11.2019 19:31


Health, 07.11.2019 19:31



Mathematics, 07.11.2019 19:31

Mathematics, 07.11.2019 19:31




Biology, 07.11.2019 19:31



Biology, 07.11.2019 19:31



