
Chemistry, 13.11.2020 21:50 paulawells11
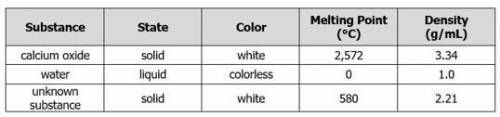
DUE NOW PEOPLEEE A teacher places a sample of calcium oxide, a white solid, into a container of water. Students observe the formation of bubbles in the container. Next, the teacher removes a sample of an unknown substance from the container. The data table compares the properties of calcium oxide, water, and the unknown substance.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
You know the right answer?
DUE NOW PEOPLEEE
A teacher places a sample of calcium oxide, a white solid, into a container of wat...
Questions


Social Studies, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

Health, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01

Mathematics, 16.10.2020 03:01


Mathematics, 16.10.2020 03:01




Spanish, 16.10.2020 03:01






Mathematics, 16.10.2020 03:01

History, 16.10.2020 03:01



