
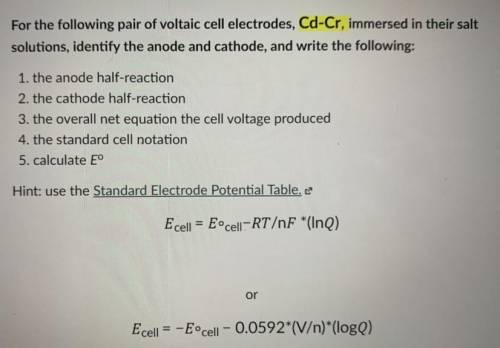
For the following pair of voltaic cell electrodes, Cd-Cr, immersed in
their salt solutions, identify the anode and cathode, and write the
following:
1. the anode half-reaction
2. the cathode half-reaction
3. the overall net equation the cell voltage produced
4. the standard cell notation
5. calculate E

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1


Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3
You know the right answer?
For the following pair of voltaic cell electrodes, Cd-Cr, immersed in
their salt solutions, identif...
Questions

History, 04.05.2021 19:50

Mathematics, 04.05.2021 19:50

Mathematics, 04.05.2021 19:50

English, 04.05.2021 19:50

Mathematics, 04.05.2021 19:50






Mathematics, 04.05.2021 19:50






Advanced Placement (AP), 04.05.2021 19:50



Biology, 04.05.2021 19:50



