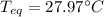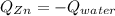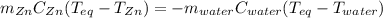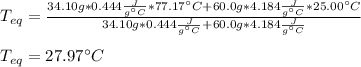Chemistry, 15.11.2020 01:00 george6871
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water shown in the interactive.
Calculate the final temperature of the water. The specific heat of nickel is 0.444 J/g °C and the specific heat of water is
4.184 J/g °C.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
A chemist heats 34.10 g of nickel to 77.17 °C, then places the metal sample in the cup of water show...
Questions




Biology, 02.08.2019 11:00



Biology, 02.08.2019 11:00


Health, 02.08.2019 11:00

History, 02.08.2019 11:00

Mathematics, 02.08.2019 11:00

History, 02.08.2019 11:00

Social Studies, 02.08.2019 11:00

Mathematics, 02.08.2019 11:00


English, 02.08.2019 11:00




History, 02.08.2019 11:00