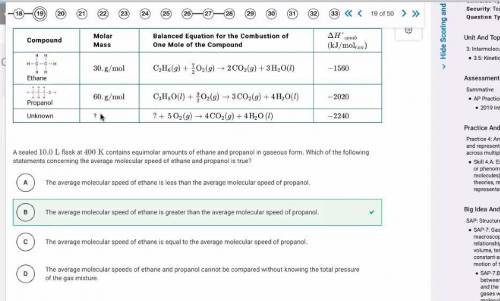
A sealed 10.0 L flask at 400 K contains equimolar amounts of ethane and propanol in gaseous form. Which of the following statements concerning the average molecular speed of ethane and propanol is true?
(A) The average molecular speed of ethane is less than the average molecular speed of propanol.
(B) The average molecular speed of ethane is greater than the average molecular speed of propanol.
(C) The average molecular speed of ethane is equal to the average molecular speed of propanol.
(D) The average molecular speeds of ethane and propanol cannot be compared without knowing the total pressure of the gas mixture.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1


Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
A sealed 10.0 L flask at 400 K contains equimolar amounts of ethane and propanol in gaseous form. Wh...
Questions


Physics, 19.02.2022 20:10

SAT, 19.02.2022 20:10

Business, 19.02.2022 20:10

Mathematics, 19.02.2022 20:10






History, 19.02.2022 20:10


History, 19.02.2022 20:20



Business, 19.02.2022 20:20




English, 19.02.2022 20:20