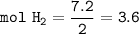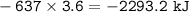Chemistry, 15.11.2020 20:40 calistaallen1734
The thermodynamic information for the following reaction is as follows:
HNO3 (g) + H2 (g) → NH3 (g) + H2O (g) △H = −637 kJ
1) Balance the chemical reaction.
2) Identify this reaction as endothermic or exothermic.
3) Calculate how much heat is released when 7.20 g of H2 reacts in this situation.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
The thermodynamic information for the following reaction is as follows:
HNO3 (g) + H2 (g) → NH3 (g)...
Questions




Biology, 14.12.2020 20:50

Mathematics, 14.12.2020 20:50

Biology, 14.12.2020 20:50


Arts, 14.12.2020 20:50

Mathematics, 14.12.2020 20:50



Business, 14.12.2020 20:50


Mathematics, 14.12.2020 20:50



Mathematics, 14.12.2020 20:50


Mathematics, 14.12.2020 20:50

Mathematics, 14.12.2020 20:50