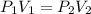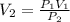Chemistry, 15.11.2020 21:40 lizzyhearts
An aerosol can contains 350.mL of gas at a pressure of 5.00 atm. What would be the volume of the gas at 2.00atm?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 17:30
Insoluble substances can dissolve in all solvents. true or false
Answers: 2

Chemistry, 23.06.2019 20:00
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant r. suppose the osmotic pressure of a certain solution is measured to be 12.atm at an absolute temperature of 317k. write an equation that will let you calculate the molarity c of this solution. your equation should contain only symbols. be sure you define each symbol other than r. equation: c=? definition of symbols: ? = 12 atm ? = 317 k
Answers: 3
You know the right answer?
An aerosol can contains 350.mL of gas at a pressure of 5.00 atm. What would be the volume of the gas...
Questions




Mathematics, 10.01.2020 10:31


Mathematics, 10.01.2020 10:31


Mathematics, 10.01.2020 10:31


Mathematics, 10.01.2020 10:31

Social Studies, 10.01.2020 10:31

History, 10.01.2020 10:31




Mathematics, 10.01.2020 10:31


Mathematics, 10.01.2020 10:31