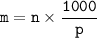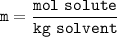Question 1 of 10
What would you need to do to calculate the molality of 10 mol of NaCl in 200
mol of water?
A. Convert the 200 mol of water to kilograms of water.
B. Convert the 200 mol of water to liters of water.
C. Convert the 10 mol of NaCl to grams of NaCl.
O D. Convert the 10 mol of NaCl to kilograms of NaCl.
SUBMIT

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Question 1 of 10
What would you need to do to calculate the molality of 10 mol of NaCl in 200
...
...
Questions



History, 22.01.2022 05:30

Arts, 22.01.2022 05:30


Mathematics, 22.01.2022 05:30





Biology, 22.01.2022 05:30

Mathematics, 22.01.2022 05:30



Computers and Technology, 22.01.2022 05:30


Mathematics, 22.01.2022 05:30


Mathematics, 22.01.2022 05:30