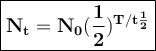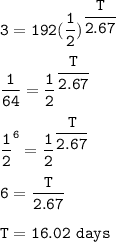Chemistry, 15.11.2020 23:10 elizabethxoxo3271
Yttrium-90, Y90 , is a radioactive isotope used in the treatment of liver cancer. The half‑life of Y90 is 2.67 days. If a dose with an activity of 192 μCi is given to a patient, how many days will it take for the activity of Y90 in the patient to reach 3.00 μCi?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

You know the right answer?
Yttrium-90, Y90 , is a radioactive isotope used in the treatment of liver cancer. The half‑life of Y...
Questions


Mathematics, 17.11.2020 01:10


Mathematics, 17.11.2020 01:10

Chemistry, 17.11.2020 01:10


Physics, 17.11.2020 01:10


Mathematics, 17.11.2020 01:10



Mathematics, 17.11.2020 01:10

Mathematics, 17.11.2020 01:10

Arts, 17.11.2020 01:10


Social Studies, 17.11.2020 01:10


English, 17.11.2020 01:10

History, 17.11.2020 01:10