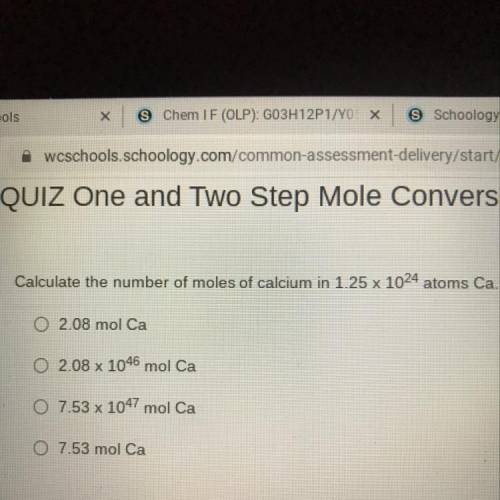
Calculate the number of moles of calcium in 1.25 x 1024 atoms Ca.
02.08 mol Ca
O 2.08 x 1046...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Questions



English, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50

Geography, 12.12.2020 16:50







Mathematics, 12.12.2020 16:50

Biology, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50