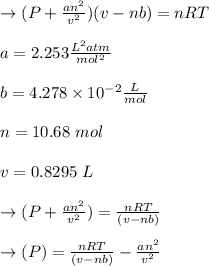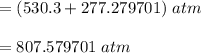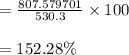Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 23.06.2019 11:30
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1
You know the right answer?
According to the ideal gas law, a 10.68 mol sample of methane gas in a 0.8295 L container at 501.9 K...
Questions

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Chemistry, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

History, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

English, 13.09.2020 09:01

Physics, 13.09.2020 09:01

Spanish, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Chemistry, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

History, 13.09.2020 09:01

History, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01

Mathematics, 13.09.2020 09:01