
Chemistry, 17.11.2020 01:00 JvGaming2001
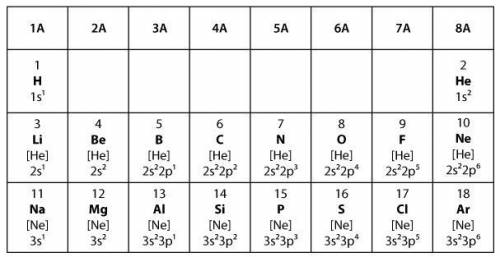
Covalent bonds form when two elements share electrons to make a complete outer shell of electrons. Ionic bonds form when one element donates one or more electrons to another element to make a complete outer shell of electrons.
Which statement best explains the type of bond that will form between two elements from group 6A in the model?
A
The two elements will form a covalent bond because both elements will share a single electron in order to have full outer shells.
B
The two elements will form a covalent bond because both elements will share a pair of electrons in order to have full outer shells.
C
The two elements will form an ionic bond because one of the elements will donate one electron to the other element in order to have full outer shells.
D
The two elements will form an ionic bond because one of the elements will donate two electrons to the other element in order to have full outer shells.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1


Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Covalent bonds form when two elements share electrons to make a complete outer shell of electrons. I...
Questions




Computers and Technology, 27.11.2019 07:31






Social Studies, 27.11.2019 07:31












