C. 9.23 x 1023 Au atoms =
moles Au
D. 125 g H3PO4 =
molecules H3PO4
E. 0.75 moles...

Chemistry, 17.11.2020 08:20 kimberlyblanco14
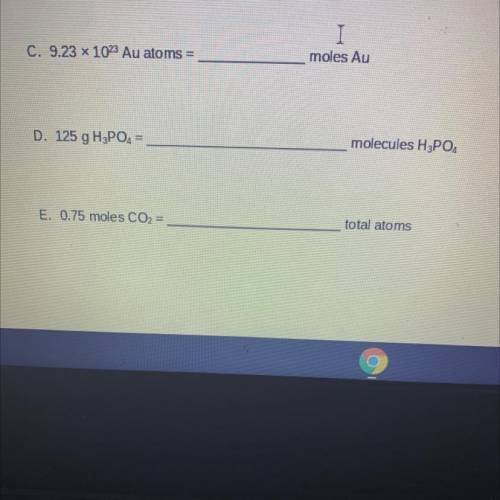
C. 9.23 x 1023 Au atoms =
moles Au
D. 125 g H3PO4 =
molecules H3PO4
E. 0.75 moles CO2 =
total atoms
help pls due in 5 min

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

You know the right answer?
Questions

Mathematics, 22.09.2020 14:01




Mathematics, 22.09.2020 14:01





English, 22.09.2020 14:01


Biology, 22.09.2020 14:01





Business, 22.09.2020 14:01


Mathematics, 22.09.2020 14:01

History, 22.09.2020 14:01



