
Chemistry, 17.11.2020 17:30 rodriguezbrian050702
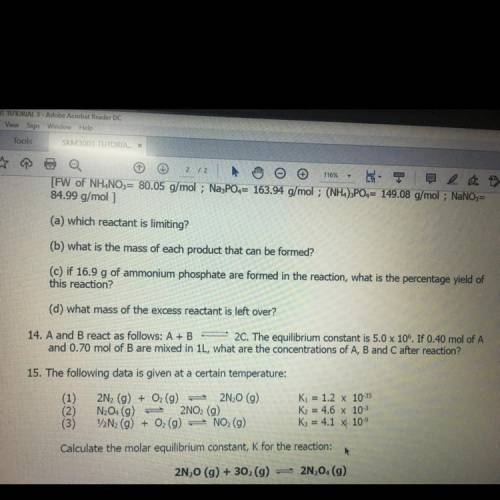
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A and 0.70 mol of B are mixed in 1L, what are the concentrations of A, B and C after reaction?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

You know the right answer?
14. A and B react as follows: A+B 2C. The equilibrium constant is 5.0 x 106. If 0.40 mol of A
and 0...
Questions


Mathematics, 23.01.2021 08:30




Biology, 23.01.2021 08:30

Mathematics, 23.01.2021 08:30


Mathematics, 23.01.2021 08:30

Physics, 23.01.2021 08:30

Mathematics, 23.01.2021 08:30


English, 23.01.2021 08:30

Business, 23.01.2021 08:30

Mathematics, 23.01.2021 08:30


Mathematics, 23.01.2021 08:30

Arts, 23.01.2021 08:30


Mathematics, 23.01.2021 08:30



