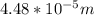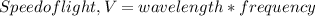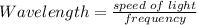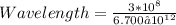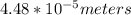Chemistry, 17.11.2020 18:30 olejlund7838
Given the speed of light as 3.0 ✕ 108 m/s, calculate the wavelength of the electromagnetic radiation whose frequency is 6.700 ✕ 1012 Hz.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

You know the right answer?
Given the speed of light as 3.0 ✕ 108 m/s, calculate the wavelength of the electromagnetic radiation...
Questions

Chemistry, 17.06.2020 03:57

History, 17.06.2020 03:57


Mathematics, 17.06.2020 03:57






Mathematics, 17.06.2020 03:57



Chemistry, 17.06.2020 03:57

History, 17.06.2020 03:57



Biology, 17.06.2020 03:57


History, 17.06.2020 03:57

Mathematics, 17.06.2020 03:57