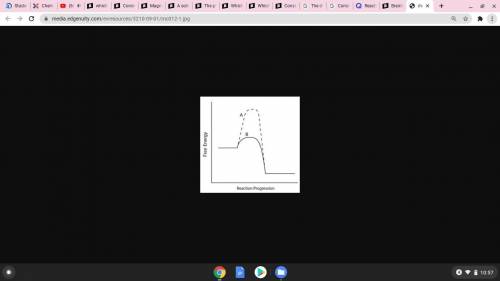
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it...


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 11:30
A) equal lines b) parallel lines c) perpendicular lines d) none of the above
Answers: 1
You know the right answer?
Questions


History, 19.03.2021 23:00


Mathematics, 19.03.2021 23:00


Arts, 19.03.2021 23:00




History, 19.03.2021 23:00


Chemistry, 19.03.2021 23:00



English, 19.03.2021 23:00



Mathematics, 19.03.2021 23:00

Mathematics, 19.03.2021 23:00

Mathematics, 19.03.2021 23:00