
Copper metal (Cu) reacts with silver nitrate (AgNO3) in aqueous solution to form Ag and Cu(NO3)2. An excess of AgNO3 is present. The balanced chemical equation is shown below.
Cu + 2AgNO3 Right arrow. Cu(NO3)2 + 2Ag
The molar mass of Cu is 63.5 g/mol. The molar mass of Ag is 107.9 g/mol. What mass, in grams, of Ag is produced from reaction of 31.75 g of Cu?
26.95
107.9
215.91
431.82

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1


Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
Copper metal (Cu) reacts with silver nitrate (AgNO3) in aqueous solution to form Ag and Cu(NO3)2. An...
Questions


Mathematics, 16.11.2020 21:40

Mathematics, 16.11.2020 21:40






Mathematics, 16.11.2020 21:40




English, 16.11.2020 21:40


Biology, 16.11.2020 21:40


English, 16.11.2020 21:40



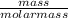
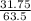 = 0.5moles
= 0.5moles 


