
Chemistry, 18.11.2020 03:20 teamroper35
The Case of the Missing Mass: Students have completed the experiment of combining vinegar and baking soda. They notice that their beginning mass in the video does not match the ending one. Use the equation(below) and the Law of Conservation of Mass to explain why the students have NOT destroyed atoms in their experiment. This is the chemical equation for the experiment:
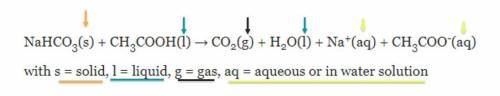

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:40
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
The Case of the Missing Mass: Students have completed the experiment of combining vinegar and baking...
Questions







History, 16.11.2020 16:40

Chemistry, 16.11.2020 16:40










Biology, 16.11.2020 16:40




