
Chemistry, 18.11.2020 04:10 shakaylaousley1997
How many grams of H2SO4 do I need to use in order to produce 3.01 moles of water? consider the equation. _Ca(OH)2 + _H2SO4 = _CaSO4 + _H20

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
How many grams of H2SO4 do I need to use in order to produce 3.01 moles of water? consider the equat...
Questions


English, 17.09.2019 10:50

Mathematics, 17.09.2019 10:50

Mathematics, 17.09.2019 10:50

Mathematics, 17.09.2019 10:50

Mathematics, 17.09.2019 10:50


Social Studies, 17.09.2019 10:50




English, 17.09.2019 10:50


Physics, 17.09.2019 10:50

Mathematics, 17.09.2019 10:50

Chemistry, 17.09.2019 10:50

Biology, 17.09.2019 10:50


Mathematics, 17.09.2019 10:50

Mathematics, 17.09.2019 10:50

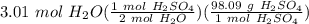 = 147.625 g H₂SO₄
= 147.625 g H₂SO₄

