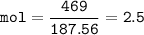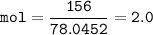Chemistry, 18.11.2020 21:10 MickeyAppleX
In this stoichiometry problem, determine the limiting reactant: Aqueous copper(II) nitrate reacts with aqueous sodium sulfide to produce aqueous sodium nitrate and copper(II) sulfide as a precipitate. In this reaction 469 grams of copper(II) nitrate were combined with 156 grams of sodium sulfide to produce 272 grams of sodium nitrate. Select all that apply.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1


Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
In this stoichiometry problem, determine the limiting reactant:
Aqueous copper(II) nitrate reacts w...
Questions




Physics, 09.04.2021 21:30

Mathematics, 09.04.2021 21:30






Mathematics, 09.04.2021 21:30


Chemistry, 09.04.2021 21:30

History, 09.04.2021 21:30

Health, 09.04.2021 21:30

History, 09.04.2021 21:30

Mathematics, 09.04.2021 21:30

Mathematics, 09.04.2021 21:30

Mathematics, 09.04.2021 21:30