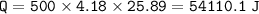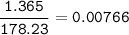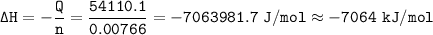When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket containing 500.0 g of water, the temperature of the water increases by 25.89°C. Assuming that the specific heat of water is 4.18 J/(g ∙°C), and that the heat absorption by the calorimeter is negligible, estimate the enthalpy of combustion per mole of anthracene.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket conta...
Questions



Chemistry, 02.10.2019 15:30

Biology, 02.10.2019 15:30


Chemistry, 02.10.2019 15:30

History, 02.10.2019 15:30





Mathematics, 02.10.2019 15:30


History, 02.10.2019 15:30

Mathematics, 02.10.2019 15:30

Mathematics, 02.10.2019 15:30

Biology, 02.10.2019 15:30

Mathematics, 02.10.2019 15:30


Biology, 02.10.2019 15:30