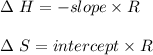A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the solubility of your salt. B. Would this initial evaporation affect the calculated solubility of your salt at each subsequent experimental saturation temperature, or just the initial temperature? Explain.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
A. Briefly explain how a loss of water by evaporation would affect the initial calculation of the so...
Questions


Mathematics, 10.11.2020 22:10

Mathematics, 10.11.2020 22:10

Mathematics, 10.11.2020 22:10

Chemistry, 10.11.2020 22:10


Mathematics, 10.11.2020 22:10







Arts, 10.11.2020 22:10

Spanish, 10.11.2020 22:10

History, 10.11.2020 22:10


Mathematics, 10.11.2020 22:10

Mathematics, 10.11.2020 22:10

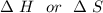 are calculated by
are calculated by 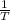 v/s lnKsp
v/s lnKsp