Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
You know the right answer?
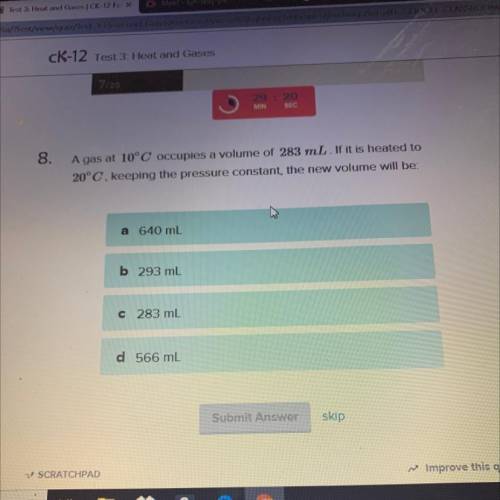
A gas at 10°C occupies a volume of 283 mL. If it is heated to
20°C, keeping the pressure constant,...
Questions

Physics, 05.06.2021 03:00




Mathematics, 05.06.2021 03:00



Physics, 05.06.2021 03:00


Physics, 05.06.2021 03:00

English, 05.06.2021 03:00


Mathematics, 05.06.2021 03:00