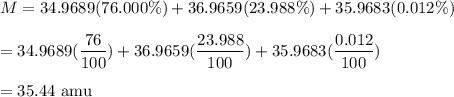Chemistry, 19.11.2020 21:00 smokemicpot
An unknown element is found to have three naturally occurring isotopes with atomic masses of 34.9689 (76.000%), 36.9659 (23.988%) and 35.9683 (0.012%) Which of the following is the unknown element?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 13:00
How many grams of oxygen gas will react completely with a block of calcium metal that is 3.0 cm by 3.5 cm by 4.2 cm, if the density of calcium is 1.55 g/ml? show all steps of your calculation as well as the final answer.
Answers: 3
You know the right answer?
An unknown element is found to have three naturally occurring isotopes with atomic masses of 34.9689...
Questions


Mathematics, 26.01.2021 20:30



Chemistry, 26.01.2021 20:30


Social Studies, 26.01.2021 20:30

Mathematics, 26.01.2021 20:30


History, 26.01.2021 20:30


Mathematics, 26.01.2021 20:30

History, 26.01.2021 20:30

Physics, 26.01.2021 20:30


Law, 26.01.2021 20:30