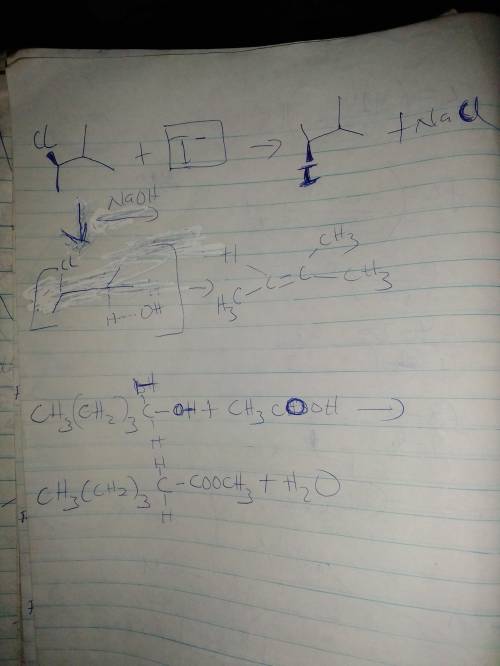Chemistry, 19.11.2020 22:50 dsperez201938
Consider the reaction of (R)-2-chloro-3-methylbutane with sodium iodide to form a
product.
1(a) Draw the reaction scheme with the correct stereochemistry (reactant + NaI → product
+ NaCl). Circle the nucleophile and draw a rectangle around the electrophile.
1(b) What is the symbol used for mechanism shown in 1(a)?
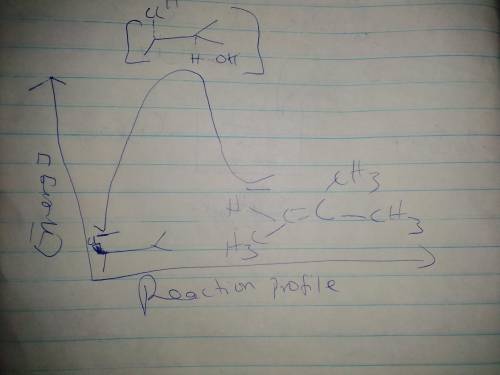
1(c) If the sodium iodide was replaced with sodium hydroxide, the product is an ALKENE. Draw a reaction MECHANISM to show how this happens.
1(d) Draw the reaction energy diagram for the reaction in 1(c) and label the activation
energy.
1(e) Using any alcohol with five carbons, and any carboxylic acid with six carbons, draw a
reaction to show how we would make an ester.
1(f) Describe the practical on esters.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1
You know the right answer?
Consider the reaction of (R)-2-chloro-3-methylbutane with sodium iodide to form a
product.
Questions

Mathematics, 19.05.2020 13:14




Business, 19.05.2020 13:14


Mathematics, 19.05.2020 13:14


Mathematics, 19.05.2020 13:14


Computers and Technology, 19.05.2020 13:14


Chemistry, 19.05.2020 13:14



Mathematics, 19.05.2020 13:14