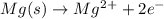Chemistry, 14.01.2020 00:31 kayvontay4
What is the oxidation half-reaction for mg(s) + zncl2(aq) mgcl2(aq) + zn(s)?
a. zn(s) --> zn^2+ + 2e-
b. mg(s) --> mg2+ + 2e-
c. zn^2+ + 2e- --> zn(s)
d. mg^2+ + 2e- --> mg(s)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

You know the right answer?
What is the oxidation half-reaction for mg(s) + zncl2(aq) mgcl2(aq) + zn(s)?
a. zn(s)...
a. zn(s)...
Questions

History, 19.09.2021 03:30

Geography, 19.09.2021 03:30


Mathematics, 19.09.2021 03:30


Advanced Placement (AP), 19.09.2021 03:40

English, 19.09.2021 03:40


Mathematics, 19.09.2021 03:40

English, 19.09.2021 03:40









Mathematics, 19.09.2021 03:40