Chemistry, 20.11.2020 17:00 queenpaige2015
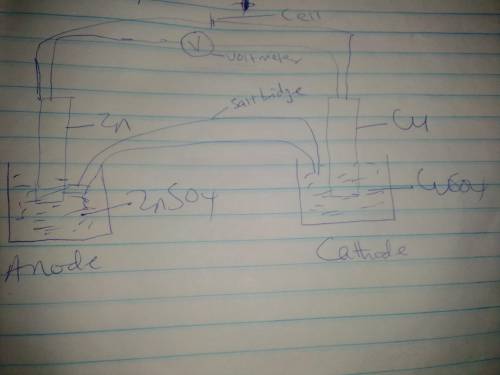
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76 V Cu^2+ + 2 e^> Cu E°cell = 0.34 V 1. Predict the standard potential of the cell at 298 K. 2. What is the minimum voltage that should be applied to the standard electrolytic cell found in question to cause zn2+ to be reduced to Zn?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Use the given half reactions to "construct" an electrolytic cell. Zn^2+ + 2 e^>Zn E°cell = -0.76...
Questions

Mathematics, 13.05.2020 21:57


Mathematics, 13.05.2020 21:57

Mathematics, 13.05.2020 21:57

Chemistry, 13.05.2020 21:57


Mathematics, 13.05.2020 21:57

Mathematics, 13.05.2020 21:57



History, 13.05.2020 21:57


History, 13.05.2020 21:57




English, 13.05.2020 21:57

Mathematics, 13.05.2020 21:57


Mathematics, 13.05.2020 21:57