
Chemistry, 20.11.2020 17:10 quincyjosiah07
Calcule la masa en gramos de cloruro de hidrógeno (HCl) que se forma cuando 5.6 L de hidrógeno molecular, medido a TPE, reacciona con un exceso de cloro molecular gaseoso. MHCl = 36.5 g/mol H2(g) + Cl2(g) → 2HCl(g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1


Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1
You know the right answer?
Calcule la masa en gramos de cloruro de hidrógeno (HCl) que se forma cuando 5.6 L de hidrógeno molec...
Questions

History, 01.12.2020 14:00


Mathematics, 01.12.2020 14:00






Mathematics, 01.12.2020 14:00




Mathematics, 01.12.2020 14:00

Mathematics, 01.12.2020 14:00





Mathematics, 01.12.2020 14:00

History, 01.12.2020 14:00

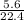 = 0.25mole
= 0.25mole 

