
Chemistry, 20.11.2020 21:50 estefaniapenalo
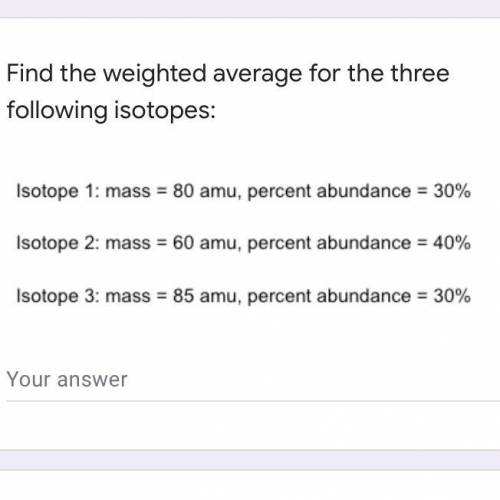
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abundance dance=30\% Isotope 2: mass = 60 amu, percent 1dance=40\% Isotope 3: mass = 85 amu, percent abundance 1dance=30\%

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Find the weighted average for the three following isotopes: Isotope 1: mass = 80 amu, percent abunda...
Questions

Mathematics, 15.07.2019 16:40


Mathematics, 15.07.2019 16:40

Arts, 15.07.2019 16:40

History, 15.07.2019 16:40


Biology, 15.07.2019 16:40

Mathematics, 15.07.2019 16:40








Chemistry, 15.07.2019 16:40



Mathematics, 15.07.2019 16:40

Mathematics, 15.07.2019 16:40



