Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
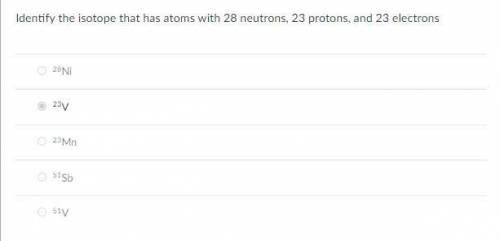
Identify the isotope that has atoms with 28 neutrons, 23 protons, and 23 electrons
A. ^28 Ni
...
...
Questions

French, 18.03.2021 21:50





English, 18.03.2021 21:50




Mathematics, 18.03.2021 21:50

Arts, 18.03.2021 21:50

Social Studies, 18.03.2021 21:50


Mathematics, 18.03.2021 21:50


Mathematics, 18.03.2021 21:50

Mathematics, 18.03.2021 21:50

English, 18.03.2021 21:50

Mathematics, 18.03.2021 21:50