

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
How much tin II fluoride will be made when reacting 45.0 grams of tin with an excess of hydrofluoric...
Questions



Social Studies, 16.01.2020 06:31

History, 16.01.2020 06:31



Mathematics, 16.01.2020 06:31

English, 16.01.2020 06:31



Mathematics, 16.01.2020 06:31

Mathematics, 16.01.2020 06:31

Social Studies, 16.01.2020 06:31


Mathematics, 16.01.2020 06:31

Biology, 16.01.2020 06:31




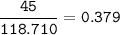
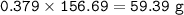 ⇒theoretical
⇒theoretical


