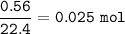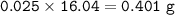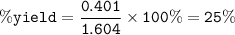Chemistry, 22.11.2020 20:50 madisongibson62
If 8.2 g of sodium ethanoate produced 560 cm3 of methane (at s. t.p.). which one of the following is the percentage yield of the reaction;
A 2.5
B 4.0
C 12.0
D 25.0

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
If 8.2 g of sodium ethanoate produced 560 cm3 of methane (at s. t.p.). which one of the following is...
Questions

Geography, 24.04.2020 06:46

Mathematics, 24.04.2020 06:46

Physics, 24.04.2020 06:46





English, 24.04.2020 06:47

Chemistry, 24.04.2020 06:47

Biology, 24.04.2020 06:47


Mathematics, 24.04.2020 06:47

Mathematics, 24.04.2020 06:47


Mathematics, 24.04.2020 06:47

Mathematics, 24.04.2020 06:47

History, 24.04.2020 06:47


Mathematics, 24.04.2020 06:47

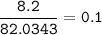
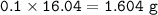 ⇒ theoretical
⇒ theoretical