
Chemistry, 22.11.2020 21:30 LtotheJ0225
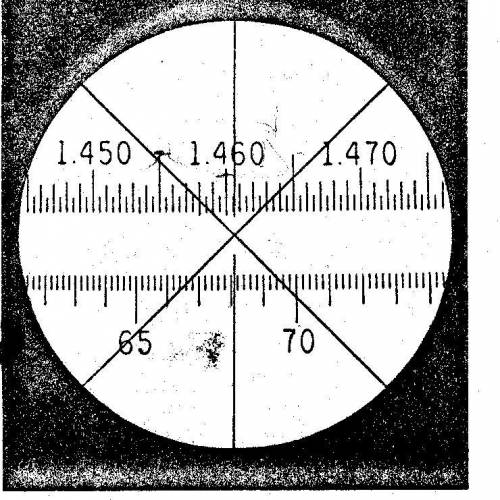
How do you determine the scale number on the refractive index? What makes this 1.4606? How did they get that 6 at the end? Like how are the last two numbers determined if the 06 weren’t there if it was just 1.46?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 11:00
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
How do you determine the scale number on the refractive index? What makes this 1.4606? How did they...
Questions

Spanish, 25.04.2021 21:30


English, 25.04.2021 21:30

History, 25.04.2021 21:30

Health, 25.04.2021 21:30

Mathematics, 25.04.2021 21:30




English, 25.04.2021 21:30

English, 25.04.2021 21:30



Business, 25.04.2021 21:30

Mathematics, 25.04.2021 21:30

Mathematics, 25.04.2021 21:30



Mathematics, 25.04.2021 21:30

Health, 25.04.2021 21:30



