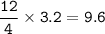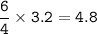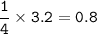Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1



Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
You know the right answer?
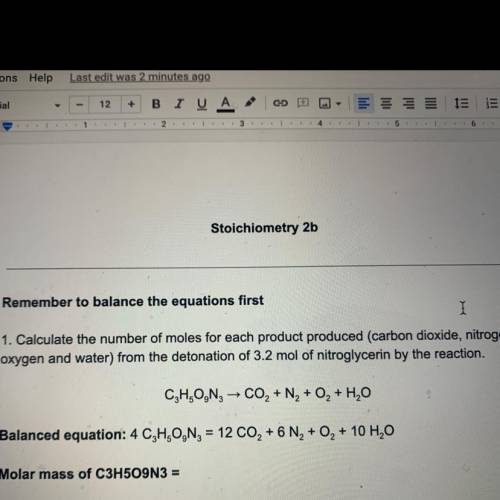
Remember to balance the equations first
1. Calculate the number of moles for each product produced...
Questions

Physics, 22.02.2021 02:30

Mathematics, 22.02.2021 02:30



Advanced Placement (AP), 22.02.2021 02:30



Biology, 22.02.2021 02:30


Mathematics, 22.02.2021 02:30

Mathematics, 22.02.2021 02:30

Mathematics, 22.02.2021 02:30

Social Studies, 22.02.2021 02:30

Mathematics, 22.02.2021 02:30

English, 22.02.2021 02:30


Mathematics, 22.02.2021 02:30


Mathematics, 22.02.2021 02:30