Chemistry, 23.11.2020 01:20 HarleyQuinn117
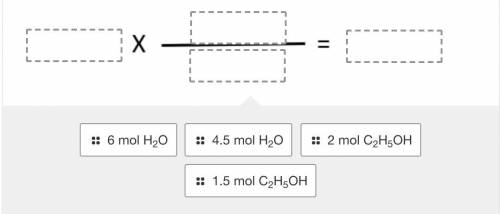
Using the balanced reaction below, drag and drop the terms into the correct location to solve the following problem:
If 1.5 moles of ethanol (C2H5OH) react, how many moles of water will be formed?
2 C2H5OH + 7 O2 --> 4 CO2 + 6 H2O

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Using the balanced reaction below, drag and drop the terms into the correct location to solve the fo...
Questions


Computers and Technology, 15.02.2022 19:00







Biology, 15.02.2022 19:00

English, 15.02.2022 19:00









English, 15.02.2022 19:00