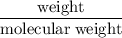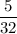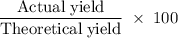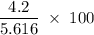Chemistry, 23.11.2020 04:10 corcoranrobert1959
A sample of 5.0 g of hydrogen gas reacts with 5.0 g of oxygen gas.
2H2(g) + O2(g) > 2H2O
Please answer the questions in a b c d and e, or at least in any of the ones you know. Thank you.


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3


Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1
You know the right answer?
A sample of 5.0 g of hydrogen gas reacts with 5.0 g of oxygen gas.
2H2(g) + O2(g) > 2H2O
Questions

History, 05.11.2020 19:50

Mathematics, 05.11.2020 19:50

Mathematics, 05.11.2020 19:50


Chemistry, 05.11.2020 19:50


Chemistry, 05.11.2020 19:50

Mathematics, 05.11.2020 19:50



Biology, 05.11.2020 19:50

Mathematics, 05.11.2020 19:50


Mathematics, 05.11.2020 19:50

English, 05.11.2020 19:50

Biology, 05.11.2020 19:50

Social Studies, 05.11.2020 19:50


Mathematics, 05.11.2020 19:50

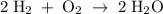
 2
2