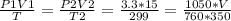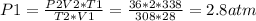2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If I raise the temperature to 350 K and lower the pressure to 1050 mmHg, what is the
new volume of the gas?
3) A gas that has a volume of 28 liters, a temperature of 65 °C, and an unknown pressure
has its volume increased to 36 liters and its temperature decreased to 35 °C. If I measure the
pressure after the change to be 2.0 atm, what was the original pressure of the gas?
work too pls

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
2) A gas takes up a volume of 15 liters, has a pressure of 3.3 atm, and a temperature of
299 K. If...
Questions

Computers and Technology, 13.12.2019 23:31









Mathematics, 13.12.2019 23:31

Health, 13.12.2019 23:31

Arts, 13.12.2019 23:31