
Chemistry, 24.11.2020 14:00 gjvyverman
II. Ionic Equations
8. Write the complete ionic and net ionic equations for the reaction below:
2 AgNO3(aq) + CaCl2(aq) → 2 AgCl + Ca(NO3)2
Complete ionic:
Net ionic:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
II. Ionic Equations
8. Write the complete ionic and net ionic equations for the reaction below:
Questions

Chemistry, 20.09.2019 01:30

Biology, 20.09.2019 01:30



Business, 20.09.2019 01:30


Spanish, 20.09.2019 01:30

Computers and Technology, 20.09.2019 01:30


Chemistry, 20.09.2019 01:30



Social Studies, 20.09.2019 01:30





Physics, 20.09.2019 01:30


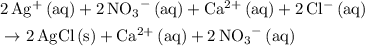 .
.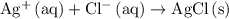 .
. 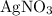 ,
, 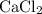 , and
, and 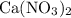 . These three salts will exist as ions:
. These three salts will exist as ions: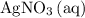 formula unit will exist as one
formula unit will exist as one 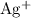 ion and one
ion and one 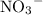 ion. Each
ion. Each 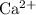 ion and two
ion and two 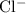 ions (note the subscript in the formula
ions (note the subscript in the formula 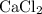 .) Each
.) Each 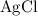 is generally insoluble in water. This salt will not form ions.
is generally insoluble in water. This salt will not form ions.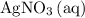 in the original equation is two,
in the original equation is two, 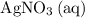 alone should correspond to two
alone should correspond to two 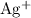 ions and two
ions and two 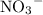 ions.
ions. 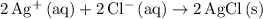 .
.

