
Chemistry, 24.11.2020 14:00 nagwaelbadawi
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction above.
18 + 25 O2 --> 16 CO2 + 18 H2O
89.6 g
17.3 g
34.5 g
46.2 g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
Calculate the mass in grams of carbon dioxide produced from 11.2 g of octane (C8H18) in the reaction...
Questions





English, 15.10.2020 17:01

Social Studies, 15.10.2020 17:01

History, 15.10.2020 17:01


History, 15.10.2020 17:01

Biology, 15.10.2020 17:01


Mathematics, 15.10.2020 17:01

History, 15.10.2020 17:01

Mathematics, 15.10.2020 17:01



Mathematics, 15.10.2020 17:01



History, 15.10.2020 17:01

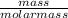
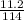 = 0.098mole
= 0.098mole 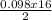 = 0.79mole of CO₂
= 0.79mole of CO₂

