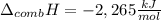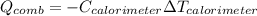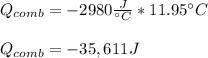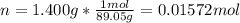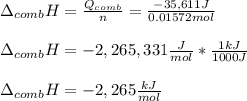Chemistry, 24.11.2020 19:20 hayleegreenwell34
A chemical compound has a molecular weight of 89.05 g/mole. 1.400 grams of this compound underwent complete combustion under constant pressure conditions in a special calorimeter. This calorimeter had a heat capacity of 2980 J °C.1 (Note that the calorimeter was made of a metal shell, a water "substitute" - a special oil, and a thermocouple). The temperature went up by 11.95 degrees.
Required:
Calculate the molar heat of combustion of the compound.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2


Chemistry, 23.06.2019 01:00
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2
You know the right answer?
A chemical compound has a molecular weight of 89.05 g/mole. 1.400 grams of this compound underwent c...
Questions


Mathematics, 17.02.2021 14:10





Mathematics, 17.02.2021 14:10

Biology, 17.02.2021 14:10



Mathematics, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10

History, 17.02.2021 14:10

Chemistry, 17.02.2021 14:10

Computers and Technology, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10

Physics, 17.02.2021 14:10

Mathematics, 17.02.2021 14:10

Health, 17.02.2021 14:10