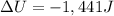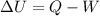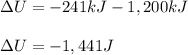Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
What is the change in internal energy ΔU for a system that releases 241J of heat and does 1.20kJ of...
Questions


English, 17.02.2021 07:50

Spanish, 17.02.2021 07:50

History, 17.02.2021 07:50

Mathematics, 17.02.2021 07:50


Chemistry, 17.02.2021 07:50


German, 17.02.2021 07:50

Biology, 17.02.2021 07:50



Mathematics, 17.02.2021 07:50



Mathematics, 17.02.2021 07:50

Computers and Technology, 17.02.2021 07:50


Mathematics, 17.02.2021 07:50

Social Studies, 17.02.2021 07:50