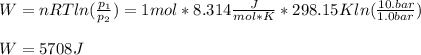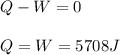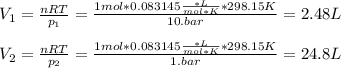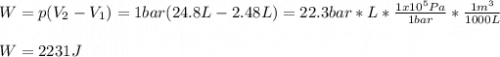Chemistry, 25.11.2020 02:30 twistedgamerhd12
One mole of an ideal gas expands reversibly and isothermally from 10. bar to 1.0 bar at 298.15K.
Required:
a. Calculate the values of w, q, âU and âH?
b. Calculate w if the gas were to have expanded to the same final state against a constant pressure of 1 bar.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
One mole of an ideal gas expands reversibly and isothermally from 10. bar to 1.0 bar at 298.15K....
Questions


Mathematics, 23.10.2020 04:01





Mathematics, 23.10.2020 04:01

Biology, 23.10.2020 04:01

Social Studies, 23.10.2020 04:01

English, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01

Biology, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01

English, 23.10.2020 04:01

Mathematics, 23.10.2020 04:01

English, 23.10.2020 04:01



Mathematics, 23.10.2020 04:01