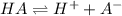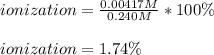Chemistry, 25.11.2020 03:30 nscarlisleh13
At equilibrium, the value of [H ] in a 0.240M solution of an unknown acid is 0.00417M . Determine the degree of ionization and the Ka of this acid.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

You know the right answer?
At equilibrium, the value of [H ] in a 0.240M solution of an unknown acid is 0.00417M . Determine th...
Questions


Biology, 15.01.2020 18:31

History, 15.01.2020 18:31



Chemistry, 15.01.2020 18:31



Social Studies, 15.01.2020 18:31

Mathematics, 15.01.2020 18:31



Mathematics, 15.01.2020 18:31



Arts, 15.01.2020 18:31

Mathematics, 15.01.2020 18:31



Biology, 15.01.2020 18:31

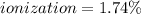
![ionization=\frac{[H^+]}{[HA]} *100\%](/tpl/images/0927/7847/7ad3f.png)