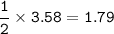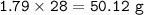Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Please Help!!
Nitrogen gas can be prepared by passing ammonia over copper(II) oxide, and the other...
Questions


Health, 05.05.2020 14:10


Biology, 05.05.2020 14:10


Biology, 05.05.2020 14:10

Chemistry, 05.05.2020 14:10

Mathematics, 05.05.2020 14:10





Mathematics, 05.05.2020 14:10

Mathematics, 05.05.2020 14:10


History, 05.05.2020 14:10

Medicine, 05.05.2020 14:10


Social Studies, 05.05.2020 14:10