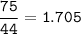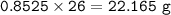Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1
You know the right answer?
Acetylene gas (C2H2) is used in welding torches. When it reacts with oxygen, it produces
carbon dio...
Questions

Mathematics, 19.10.2020 23:01


Medicine, 19.10.2020 23:01

Arts, 19.10.2020 23:01


Biology, 19.10.2020 23:01



English, 19.10.2020 23:01


Physics, 19.10.2020 23:01


Mathematics, 19.10.2020 23:01


Mathematics, 19.10.2020 23:01





Advanced Placement (AP), 19.10.2020 23:01