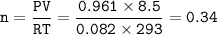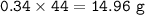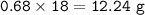Chemistry, 26.11.2020 03:00 kell22wolf
If 8.50 L of natural gas, which is essentially methane (CH4), undergoes complete combustion at 730 mm Hg and 20 degrees C, how many grams of each product are formed?
Grams of CO2=
Grams of H2O=

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
If 8.50 L of natural gas, which is essentially methane (CH4), undergoes complete combustion at 730 m...
Questions

Mathematics, 28.10.2020 19:20


Mathematics, 28.10.2020 19:20

English, 28.10.2020 19:20

English, 28.10.2020 19:20

Mathematics, 28.10.2020 19:20




Health, 28.10.2020 19:20



Mathematics, 28.10.2020 19:20

Advanced Placement (AP), 28.10.2020 19:20

Mathematics, 28.10.2020 19:20