Chemistry, 26.11.2020 19:20 madelynlittle5399
The temperature of a sample of CH4 gas (10.34 g) in a 50.0 L vessel at 1.33 atm is °C.
a.
984
b.
-195
c.
-1260
d.
-195

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
The temperature of a sample of CH4 gas (10.34 g) in a 50.0 L vessel at 1.33 atm is °C.
a.
Questions



History, 29.03.2021 23:20

Mathematics, 29.03.2021 23:20



Mathematics, 29.03.2021 23:20

Chemistry, 29.03.2021 23:20










Mathematics, 29.03.2021 23:20

History, 29.03.2021 23:20

Mathematics, 29.03.2021 23:20

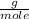 , that is, the mass present in one mole of an element or compound, the number of moles that 10.34 grams contains is calculated as:
, that is, the mass present in one mole of an element or compound, the number of moles that 10.34 grams contains is calculated as: