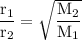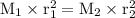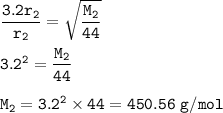Chemistry, 26.11.2020 23:10 holman6884
Explain how the rate of diffusion of a gas is related to its molar mass. Carbon dioxide gas (CO2) effuses 3.2 times faster than an unknown gas. Determine the molar mass of the unknown gas. Show your work or explain your answer, giving specific values used to determine the answer

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
Explain how the rate of diffusion of a gas is related to its molar mass.
Carbon dioxide gas (CO2) e...
Questions

Mathematics, 29.03.2021 23:50

Chemistry, 29.03.2021 23:50

Social Studies, 29.03.2021 23:50

Mathematics, 29.03.2021 23:50


Chemistry, 29.03.2021 23:50

Mathematics, 29.03.2021 23:50



Mathematics, 29.03.2021 23:50

English, 29.03.2021 23:50

Mathematics, 29.03.2021 23:50

Social Studies, 29.03.2021 23:50

History, 29.03.2021 23:50






Mathematics, 29.03.2021 23:50