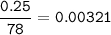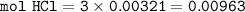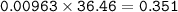Chemistry, 27.11.2020 02:00 carrieaj08
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in antacid tablets neutralizes hydrochloric acid in the stomach. A tablet containing 0.25 g of
aluminum hydroxide is ingested by a patient with 0.88 g of hydrochloric acid in their stomach. Is
this tablet sufficient to neutralize the acid in the patient's stomach? Explain using stoichiometric
calculations. [4 marks]
I

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3


Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

You know the right answer?
2. Al(OH),(s) + 3 HCl(aq) à 3 H2O(l) + AlCl3(aq). This reaction shows how aluminum hydroxide
in ant...
Questions



History, 10.07.2019 22:00

Mathematics, 10.07.2019 22:00



Advanced Placement (AP), 10.07.2019 22:00


Health, 10.07.2019 22:00

English, 10.07.2019 22:00


History, 10.07.2019 22:00



English, 10.07.2019 22:00





Physics, 10.07.2019 22:00