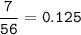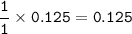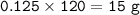Chemistry, 27.11.2020 14:00 whitakers87
Sulfur dioxide is an atmospheric pollutant.
Sulfur dioxide pollution is reduced by reacting calcium oxide with sulfur dioxide to
produce calcium sulfite.
CaO + SO2 → CaSO3
7.00 g of calcium oxide reacts with an excess of sulfur dioxide.
Relative atomic masses (Ar): O = 16 S = 32 Ca = 40 Calculate the mass of calcium sulfite produced


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
Sulfur dioxide is an atmospheric pollutant.
Sulfur dioxide pollution is reduced by reacting calcium...
Questions

Biology, 14.07.2019 07:00



English, 14.07.2019 07:00





Chemistry, 14.07.2019 07:00


English, 14.07.2019 07:00

History, 14.07.2019 07:00


Mathematics, 14.07.2019 07:00